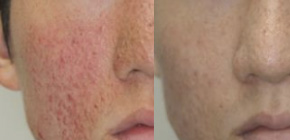
Topical Rapamycin for Facial Angiofibromas in a Child with Tuberous Sclerosis Complex (TSC): A Case Report and Long-Term Follow-up. - Abstract - Europe PMC

Keratosis pilaris rubra successfully treated with topical sirolimus: Report of a case and review of the literature - Eckburg - 2022 - Pediatric Dermatology - Wiley Online Library
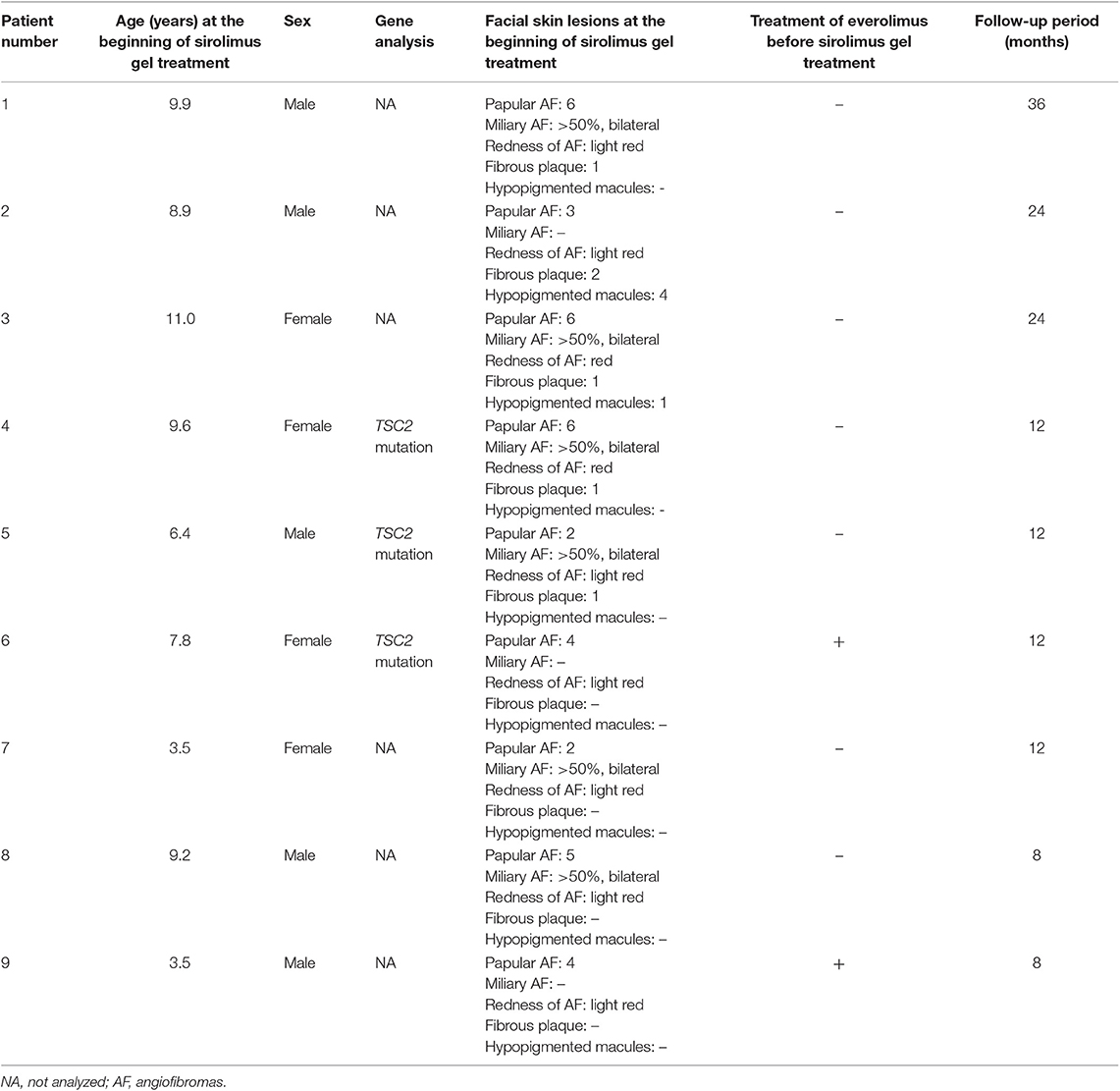
Frontiers | Early Sirolimus Gel Treatment May Diminish Angiofibromas and Prevent Angiofibroma Recurrence in Children With Tuberous Sclerosis Complex

Nobelpharma America on X: "We're excited to announce that HYFTOR™ (sirolimus topical gel) 0.2% is now approved by the FDA! Learn more: https://t.co/VonIUt4ZVi https://t.co/P1WjZ6J4Xs" / X

Figure 3 from Comparative Effects of Topical 0.2% Sirolimus for Angiofibromas in Adults and Pediatric Patients with Tuberous Sclerosis Complex | Semantic Scholar

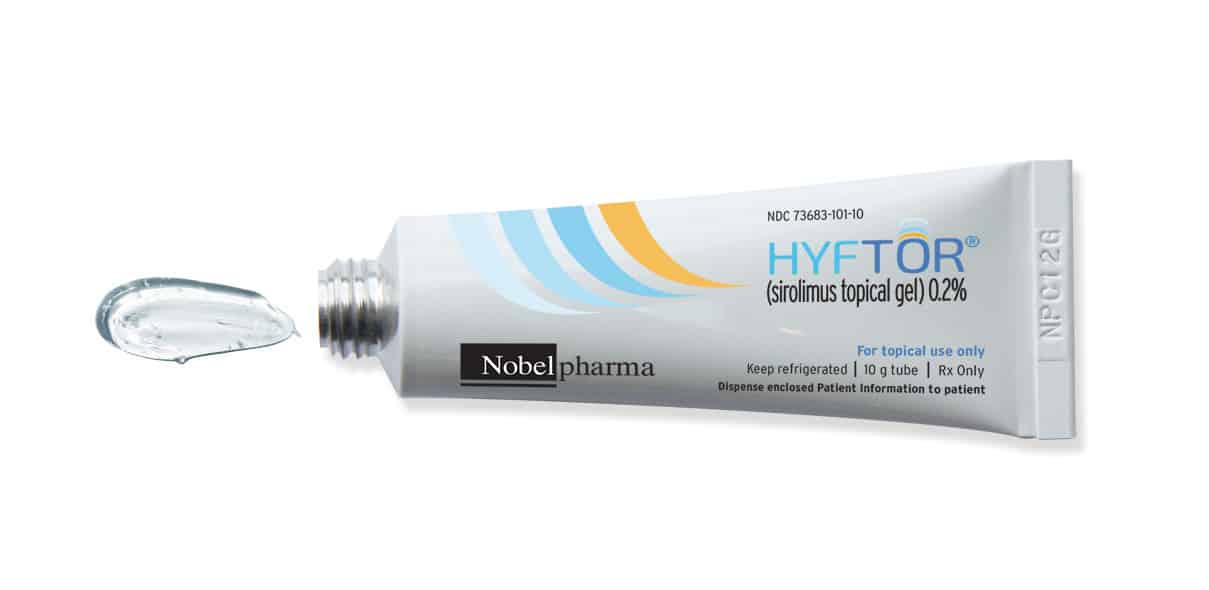
HYFTOR™ (sirolimus topical gel) 0.2%, the first FDA-approved topical treatment for facial angiofibroma associated with tuberous sclerosis, is now available in the US

Topical rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: Follow up of a pilot study and promising future directions - Tu - 2014 - Australasian Journal of Dermatology - Wiley Online Library

Polymeric micelle formulations for the cutaneous delivery of sirolimus: A new approach for the treatment of facial angiofibromas in tuberous sclerosis complex - ScienceDirect

These highlights do not include all the information needed to use HYFTOR™ safely and effectively. See full prescribing information for HYFTOR. HYFTOR™ (sirolimus topical gel) Initial U.S. Approval: 1999

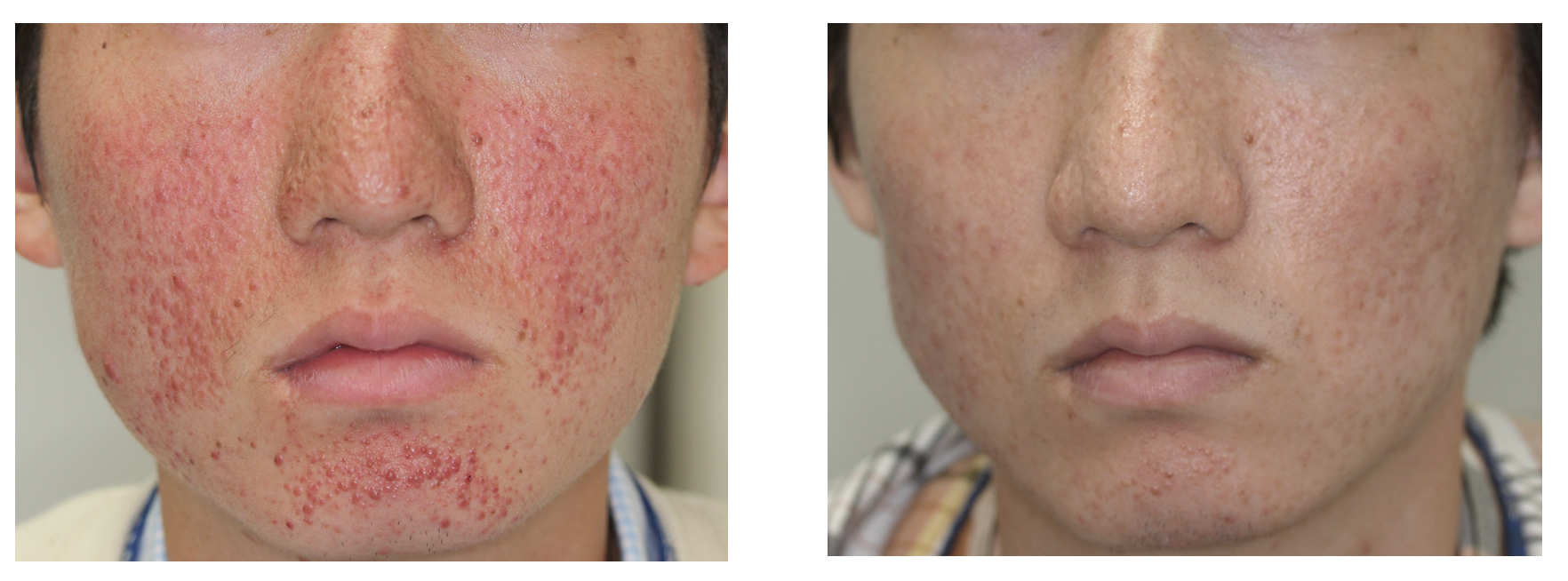
Efficacy and Safety of Topical Sirolimus Therapy for Facial Angiofibromas in the Tuberous Sclerosis Complex: A Randomized Clinical Trial | Semantic Scholar
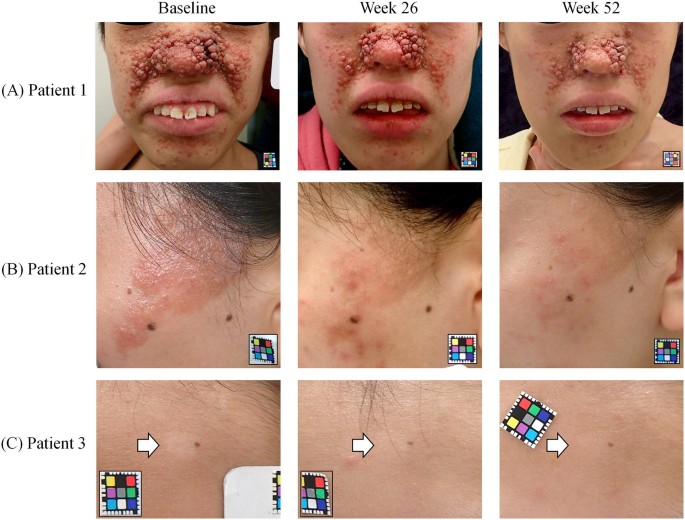
Safety and Efficacy of the Sirolimus Gel for TSC Patients With Facial Skin Lesions in a Long-Term, Open-Label, Extension, Uncontrolled Clinical Trial | Dermatology and Therapy
![PDF] Sirolimus Gel Treatment vs Placebo for Facial Angiofibromas in Patients With Tuberous Sclerosis Complex: A Randomized Clinical Trial | Semantic Scholar PDF] Sirolimus Gel Treatment vs Placebo for Facial Angiofibromas in Patients With Tuberous Sclerosis Complex: A Randomized Clinical Trial | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/54f9e54723a39a55b43d74e366e743df79c21bd7/6-Figure3-1.png)